Labeling & serialization
An efficient, transparent supply chain starts with every unit of your product. Our serialized packaging solutions give you the end-to-end visibility you need.
An efficient, transparent supply chain starts with every unit of your product. Our serialized packaging solutions give you the end-to-end visibility you need.
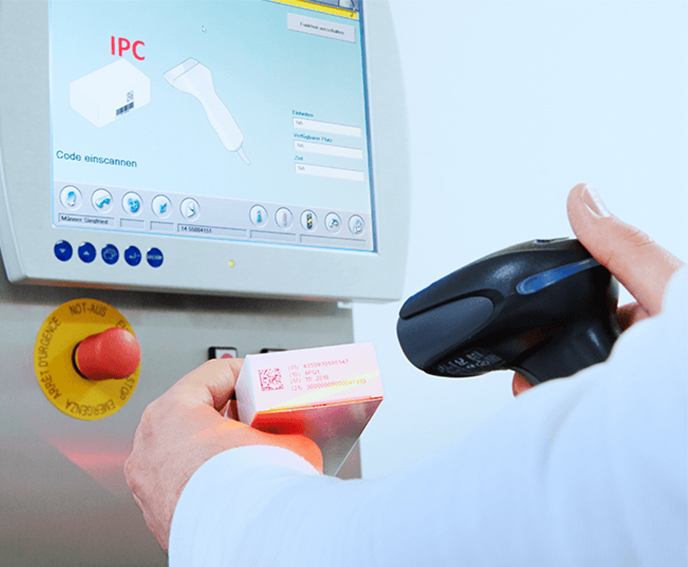

In today's global healthcare market, you need to know where your product has been and where it's headed at every point in your supply chain. At Vetter, we're experts at building systems that give you full visibility into your product's whereabouts.
Our track and trace solutions take the guesswork and estimates out of your supply. With our distribution systems, you can pinpoint your product’s location and follow its progress at every step from batch release to point of care.
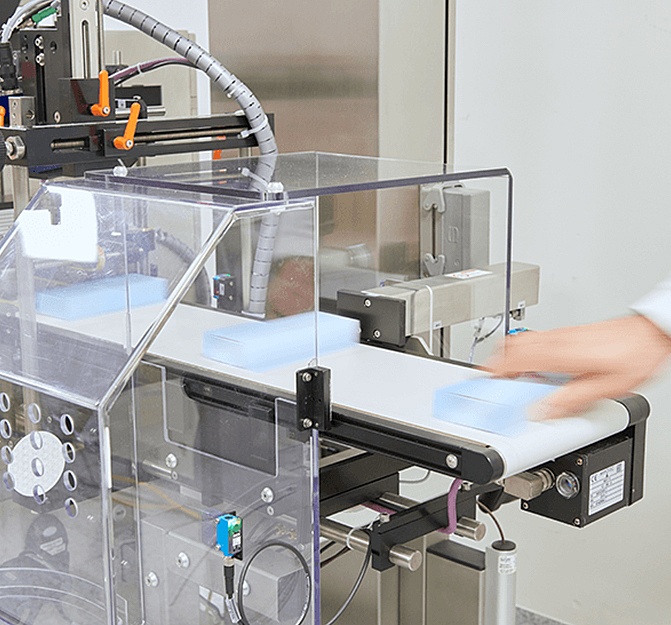
Serialization standards can vary significantly by region, so we offer a range of flexible options to help you comply with specific regulatory requirements for your target markets.
Our experts will work closely with you to develop the right approach to serializing each sales unit and aggregating them in cases and/or palettes. This parent/child relationship helps ensure we’re able to identify the exact contents of your product packages at any point in their journey to the patient.
Information about your serialized product can be conveyed through multiple data exchange formats and protocols customized for your track-and-trace program:
We protect data from your product's track-and-trace program in two different ways:
Whether your product is aggregated in a carton, case, or pallet, we offer a range of linear and 2D matrix code options to meet different supply chain needs. Depending on the level of security you require, serial numbers can be generated sequentially or randomized.
Depending on your product and compliance needs, we can integrate sampling and testing procedures in two different ways:
Our track-and-trace systems provide detailed product data from start to finish: from serial number commissioning reports immediately after packaging, to 100% documentation of all decommissioned serial numbers. You'll receive reports on all shipping events as well.