Expert device assembly & packaging services
Autoinjectors, pens, and other patient-friendly formats are reshaping the market for injectable drug products. Learn more about the strategic, technical, and packaging support we provide.
Autoinjectors, pens, and other patient-friendly formats are reshaping the market for injectable drug products. Learn more about the strategic, technical, and packaging support we provide.
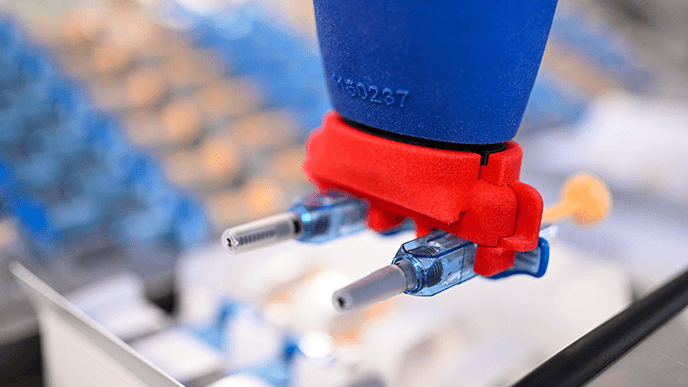

At Vetter, our experts can see the full technical, strategic, and operational context for your device assembly project. Find out how we build assembly processes that factor in your primary packaging, product characteristics, and secondary packaging plans.

We assemble multiple patient-centric delivery devices formats, as well as a range of components designed to support the usability and integrity of syringe-based products.
Explore our available options and learn more about how we can help you deliver the safe, convenient self-injection experience today’s patients expect.

With our packaging options and production expertise, your therapy will head to market in a format that both protects your product and supports premium value.
Take a closer look at our range of customizable options, including protective blister packs and sustainable all-paper cartons.

In today’s complex global markets, maximizing supply chain visibility is more important than ever. We offer state-of-the-art logistics solutions that help you pinpoint the location of every product unit and precisely track its progress from batch release to the point of care.

Manufacturing today’s drug delivery devices takes technical expertise, deep experience, and state-of-the-art machinery – and you’ll find them all in Ravensburg, Germany. Our dedicated facility is where innovative injectable therapies transform into high-value combination products.
Steps from our aseptic filling lines, this world-class site is equipped with 8 automated packaging lines, 5 manual lines, and 6 standalone labeling/assembly machines. It provides cGMP-compliant services for markets around the world.
We currently assemble and package a wide range of injectable product formats, including:
We also offer a variety of configurations, including top-loading, side-loading, blistering, multi-product cartons, and more. Please contact our team to discuss the options available for your injectable product.
We currently handle all our customers’ device assembly and packaging projects at our dedicated, state-of-the-art facilities in southern Germany.
Centralizing this service provides multiple benefits:
Yes! We offer a range of packaging options that use 100% recyclable materials. Get in touch to learn about the configurations that may be right for your injectable product.
All products assembled or packaged in our facilities must be validated on our manufacturing equipment and processed by qualified personnel. Reach out to our team to discuss the feasibility of these requirements for your product and selected format.