
Vetter Development Service’s lead scientific experts showcased insights into recent innovations at the Analytical Science Laboratory.
As always, the conference, with its wide range of topics, offered valuable feedback and fresh ideas to inspire our ambitious scientific endeavors.
We asked two Vetter experts who attended the conference to share their key takeaways:

Dr. Melanie Zerulla-Wernitz
“The PharmaLab Congress is an invaluable platform for engaging with leading experts on the latest regulatory requirements in key areas such as analytical method lifecycle, equipment qualification, and the transfer of analytical procedures. Additionally, the congress offers a broader perspective, e.g. with a glimpse on AI-supported in vitro and in silico drug development methods and or new insights in the future of IT security in modern laboratories. I was especially proud to present our latest process optimizations and initial steps toward automating sub-visible particulate matter testing—an important advancement for the Center of Excellence Particulate Matter at Vetter’s Analytical Science Laboratory.”
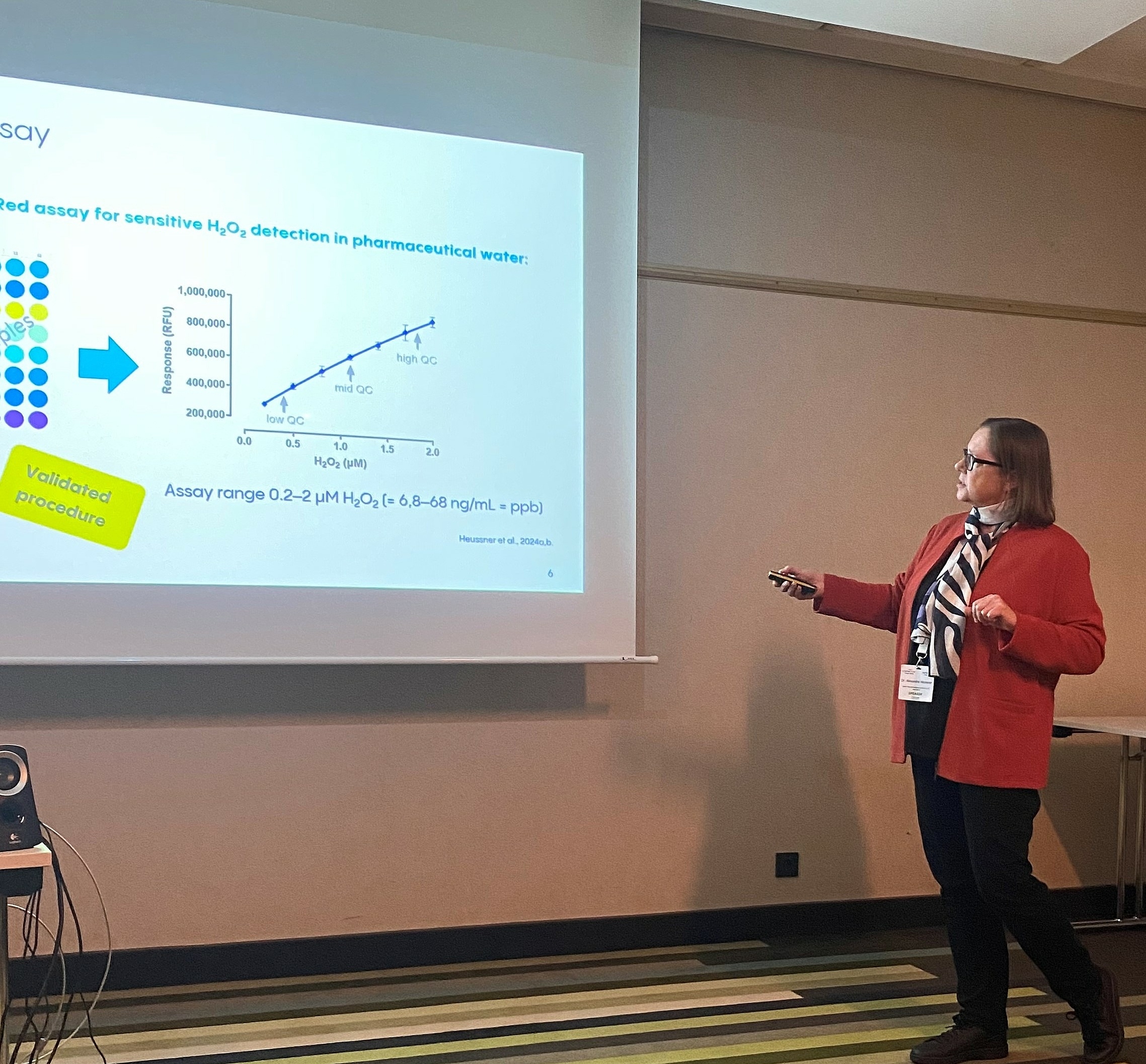
Dr. Alexandra Heussner
“As a frequent attendee of the PharmaLab Congress my expectations were once again surpassed. I had the opportunity to present and discuss how Vetter’s Analytical Science Laboratory manages the transfer of a validated, instrument-sensitive analytical procedure to a new analytical instrument. Additionally, expert presentations at the conference reinforced our practices in areas such as instrument qualification and the development and validation of analytical procedures. This valuable feedback makes the conference an essential component in our ongoing pursuit to enhance our scientific work at Vetter’s Analytical Science Laboratory.”


