Vetter Development Service Rankweil
(VDS-R)
Our dedicated clinical development facility in Austria is located just an hour’s drive from our headquarters and commercial manufacturing hub in southern Germany.
Our dedicated clinical development facility in Austria is located just an hour’s drive from our headquarters and commercial manufacturing hub in southern Germany.

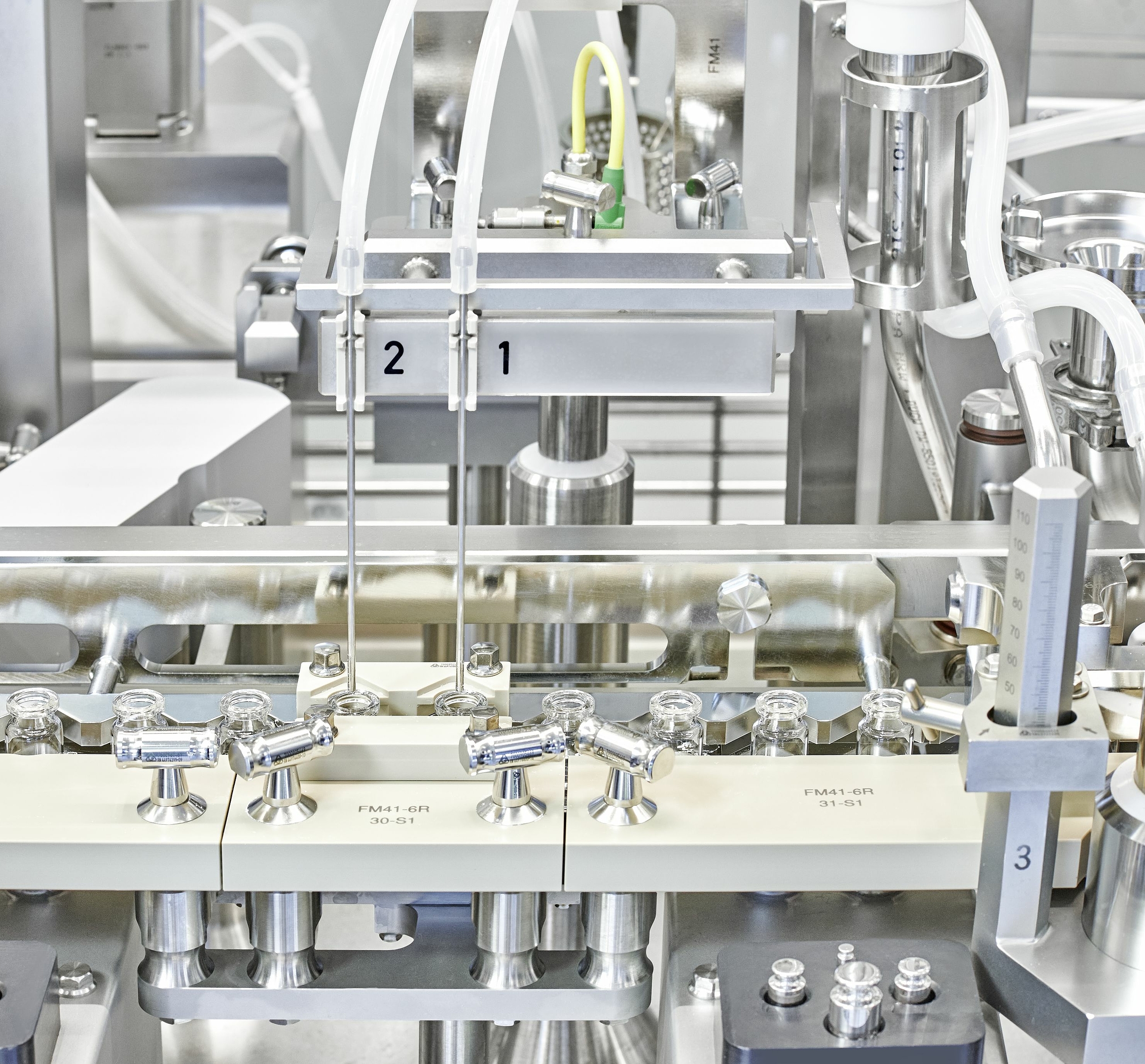
The latest addition to our global site network, VDS-R is optimized for parenteral molecules in the earliest phases of clinical development. This 100,000 ft2 site offers the resources needed for efficient early-stage clinical manufacturing including chemical analysis, microbiology labs, material preparation and compounding functions.
Key features & resources: