In today’s challenging capital environment, early-stage drug developers are under increasing pressure to launch clinical trials as swiftly and efficiently as possible. With funding scarce, these up-and-coming companies can rarely afford costly setbacks in their clinical development programs.
More than ever, these innovators need strategic thinking and expert support for the launch of their in-human trials. During that process, the right Contract Development and Manufacturing Organization (CDMO) can be more than a valuable service provider – they can be a vital partner on this critical early stage of the product development journey.
Here are five crucial ways a clinical trials CDMO can help support the successful launch of new in-human studies.
Don't want to read the full article? You can also watch our latest webcast in which we take an in-depth look at how to get your first clinical batch right:

1. Planning production of your clinical trial material (CTM)
For injectable drug products, the transition from benchtop production to cGMP clinical manufacturing is a vital development step – and one that involves much more than simply scaling production of an active pharmaceutical ingredient (API).
This important step often marks multiple firsts for a drug product candidate:
- The first time a drug substance needs to be produced in a human-ready dose form
- The first time it will be produced in a professional manufacturing environment, instead of a lab
- The first time a partner will need to leverage and interpret your analytical testing methods
- The first time the product’s manufacturing processes will be reviewed and approved by regulators
Successfully navigating each of these groundbreaking steps takes experience, careful planning, and technical expertise – especially with sterile injectables. Here are some of the key ways a clinical trial CDMO can help support that success:
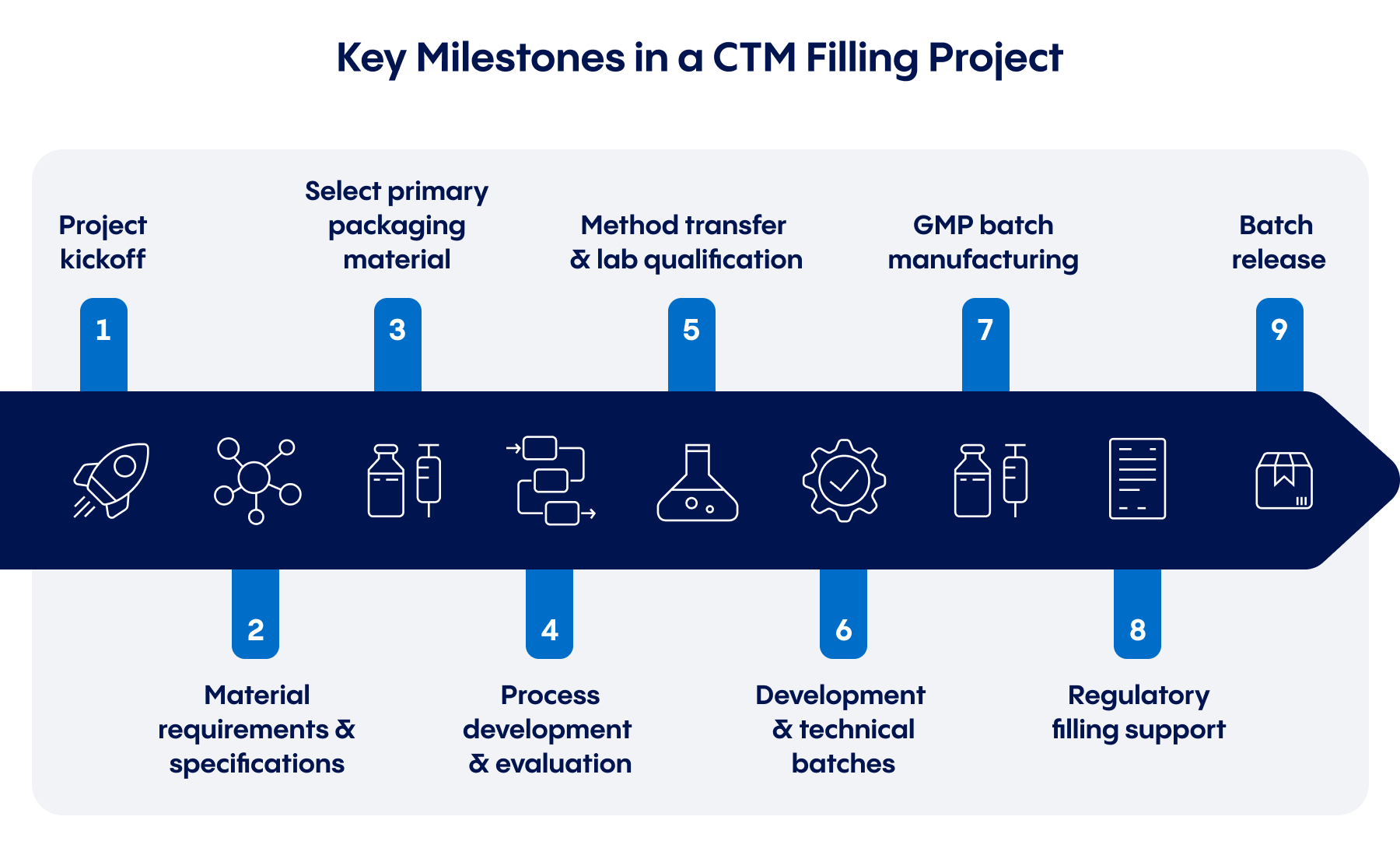
- Identifying development needs: The right CDMO for your clinical trials can help assess the development work completed so far, pinpoint necessary next steps, and help determine realistic timelines for CTM that meet the high standards required for in-human use.
- Mapping the regulatory pathway: Manufacturing organizations experienced with clinical trials have a deep understanding of what regulators’ expectations for different drug substances, delivery formats, and more. The right partner can provide valuable insights that help proactively support compliance.
- Evaluating the API: An expert clinical trial CDMO will be able to determine how much API will be needed to produce a target amount of CTM, but also how that API will need to be handled, stored, and protected during clinical manufacturing.
- Building a project timeline: Working side-by-side with an experienced manufacturing partner can help a drug developer create an informed, strategic roadmap for their CTM project. This is key for both scheduling and budgeting accuracy.
While some milestones may vary depending on a product’s formulation, packaging, and other factors, most injectable CTM projects will follow a flow similar to the one above. A strategic manufacturing partner can help their customer manage each step as efficiently as possible, and keep Phase I, Phase II, and Phase III trials on track for timely initiation.
2. Assessing your API for manufacturing readiness

For drug developers, the pivot from lab to clinic also brings a significant shift in focus: from the science of their compound or molecule to how it can be produced at the scale and quality required for clinical trials.
To make that transition safely and efficiently, an expert partner will need to evaluate and understand many new dimensions of the API – including many attributes that define how it must be handled in a professional aseptic filling environment.
If they’re familiar with the complexities of sterile manufacturing, that organization will want to thoroughly assess:
- Product formulation: A knowledgeable partner can help determine if the product’s current formulation is optimal for a Phase I, II or III clinical trial, and propose strategic alternatives if needed.
- Sensitivities: The right manufacturing partner can also help identify factors that may impact the integrity of an API (e.g., light, temperature, turbidity) and plan production processes accordingly.
- Handling methods: Protecting both product integrity and personnel safety will be top priorities for any CDMO, starting with a detailed assessment of how an API needs to be prepared, stored, and processed in a sterile manufacturing facility.
- Sourcing: To keep a CTM project on track, an experienced partner will build a detailed catalog of the resources and materials needed to prepare a drug product – as well as where and how they can be procured at the necessary scale.
Each of these considerations can be critical to a drug product’s transition from the lab to a clinical manufacturing environment – especially during early phase product manufacturing.
The right clinical production CDMO can help spot processability challenges and process needs early on, protecting both your precious API and vital milestones for your clinical trials.
3. Selecting an optimal container for clinical trials… and beyond

Selecting a primary container is one of the most important early decisions for an injectable drug product. But while vials may be the traditional default option – given their well-established manufacturing processes and regulatory pathways – several market dynamics are driving drug developers to broaden their outlook earlier in the product life cycle.
When it comes to exploring other container options, a CDMO who knows parenteral clinical trials can provide insights on several increasingly important factors:
- Evolving patient needs: With self-administered formats increasingly in-demand, a strategic partner can help determine when it makes the most sense to transition to a syringe- or cartridge-based format.
- Deal preparation: A market-savvy partner will also know what potential licensees are looking for in an injectable acquisition, and can help emerging drug developers plan their CTM projects accordingly.
- Shifting life cycle responsibilities: With capital growing scarce, a forward-thinking manufacturer can help their customer proactively plan for costly milestones – like a patient friendly format – that may be pushed down by future licensees or purchasing organizations.
All three of these considerations can inform primary container selection in valuable ways, especially with input from a clinical manufacturing partner who has helped guide many injectable products through Phase I – III trials.
That partner’s insights can help lead their customer to a smart selection that supports multiple downstream successes – from establishing clinical value to securing patient preference and advantageous deal terms.
4. Transferring and adapting your analytical methods
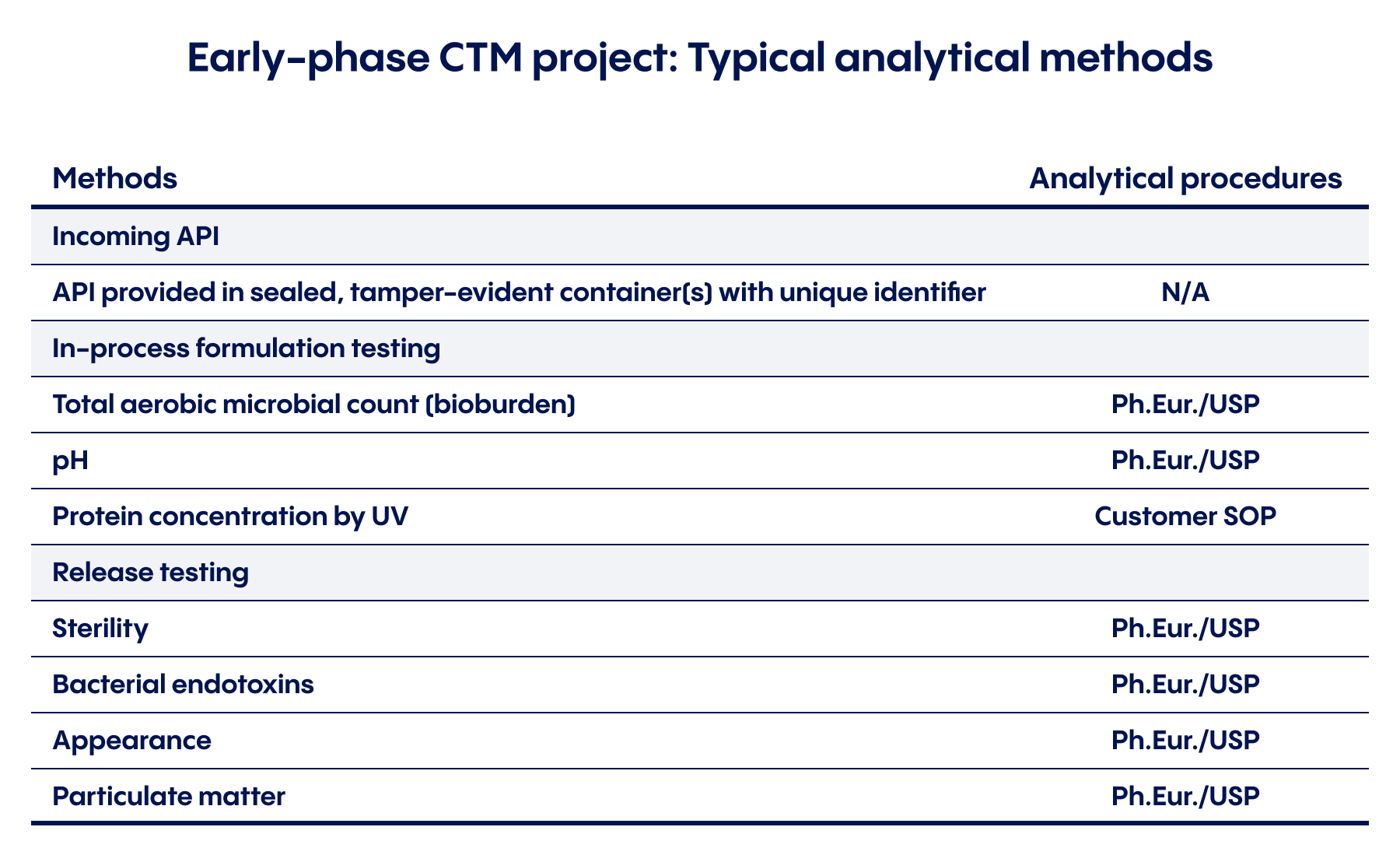
Another key step is to ensure that your product can be manufactured to required specifications and that your development and manufacturing partner has the ability to accurately measure and monitor the quality throughout the process:
Method evaluation: From identity, to stability, potency, bioburden, and more, a CDMO can help determine how specialized analytical methods may need retooling for a new, larger-scale testing setup.
SOP adaptation: An expert drug manufacturer can carefully evaluate Standard Operating Procedures (SOPs) for drug testing, and guide the customer through any adjustments that may need to be made for clarity or feasibility.
Quality by design: To prepare methods for regulatory oversight, a drug manufacturer for clinical trials can help further define critical quality attributes, process parameters, material attributes, and more – critical factors in both cGMP compliant and ICH-aligned manufacturing processes.
Here’s a closer look at some of the essential testing methods that these evaluations will typically focus on for an early-stage CTM project. A contract manufacturer experienced with clinical trials can help assess each of these methods and procedures, and identify the most effective way to transfer them from R&D lab to analytical testing lab.
5. Managing your CTM batch fill
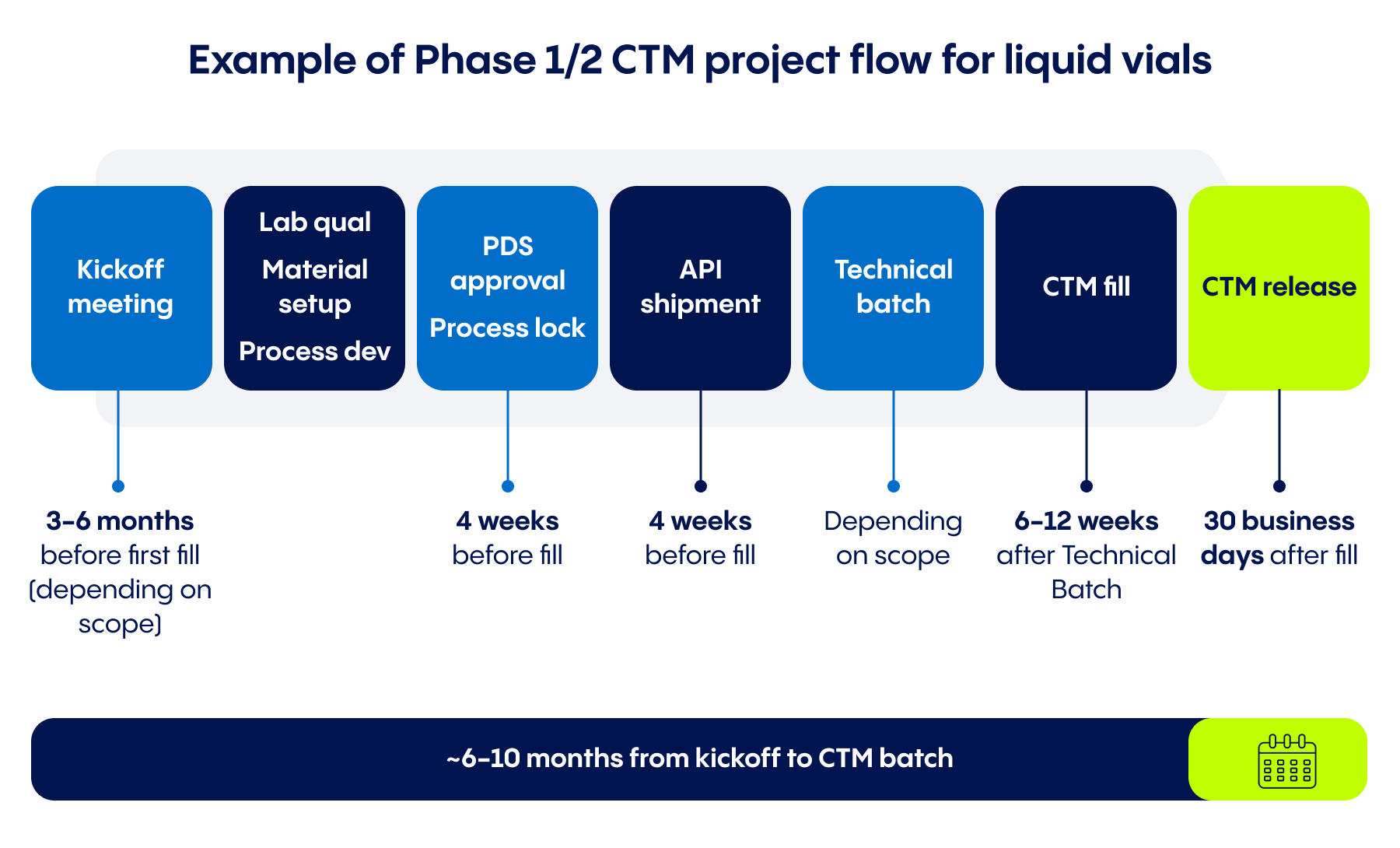
Once many key factors have been considered – including schedules, API, container type, analytics, and more – a manufacturing organizations can move ahead with the specialized process of filling the product needed for clinical trials. An optimized approach to this critical batch can help mitigate many risks, streamline many processes, and keep clinical trials on track for launch. .
To support the success of that project, a CDMO can help guide many key operational decisions and processes such as:
- Timeline planning: A CTM filling run has many interdependent steps that a partner can help translate into a feasible, efficient timeline for batch release. In the infographic above you can see what those steps can look like for a Phase I or Phase II clinical batch manufacturing project for liquid CTM in vials.
- API quantity: To protect both timelines and budgets, a CDMO can also help determine the volume of API that will be needed to complete a CTM filling project – factoring in line loss, overages, destructive sampling for quality testing, and other considerations.
- Technical runs: While not required, this preparatory step can be a valuable way to dial in filling processes and mitigate some potential risks before committing precious API to full production cycle. In some cases, technical runs can even be completed using placebos to further preserve available API.
- Cold chain logistics: For frozen CTM, a manufacturer familiar with clinical trials can help map out the steps needed to safely distribute doses to destination clinics.
How to find the CDMO you need for your clinical trials

As each of these factors shows, a contract manufacturer can be more than just an operational service provider – they can be a key strategic partner at a critical stage in an injectable product’s development journey.
When selecting a CDMO for your clinical trials, there are a number of important considerations to discuss and evaluate to determine if a manufacturer is a good fit for both your product and your project goals. The right partner should not only offer extensive technical capabilities, deep experience, and state-of-the-art facilities, but also key advantages such as:
- Specialized expertise: Know-how with your specific product type, substance class, and delivery format can all help streamline your CTM project.
- Dedicated service: Assess their responsiveness, adaptability, and overall commitment to your project – especially critical for early-stage companies relying on this external support.
- Clear communication: Seek out a partner that demonstrates clarity and proactive communication about project expectations, timeline management, and any potential setbacks.
- Forward thinking: Does the strategic manufacturing partner have an eye toward your development goals and licensing plans? They should see where your product is headed and help you make early decisions that set you up for long-term success beyond initial clinical trials.
To assess whether a CDMO will be a good fit for your product and clinical trials, consider some of these questions early in the vetting process. If they can answer “yes” to all of them, they may be a strong choice for your clinical batch manufacturing project.


